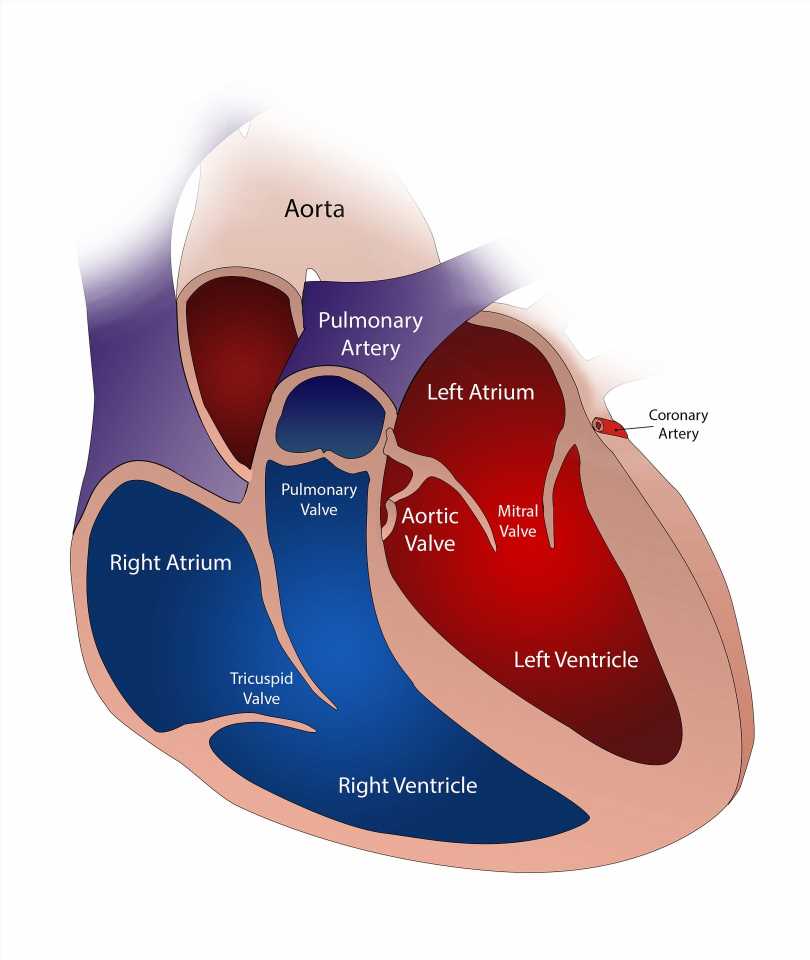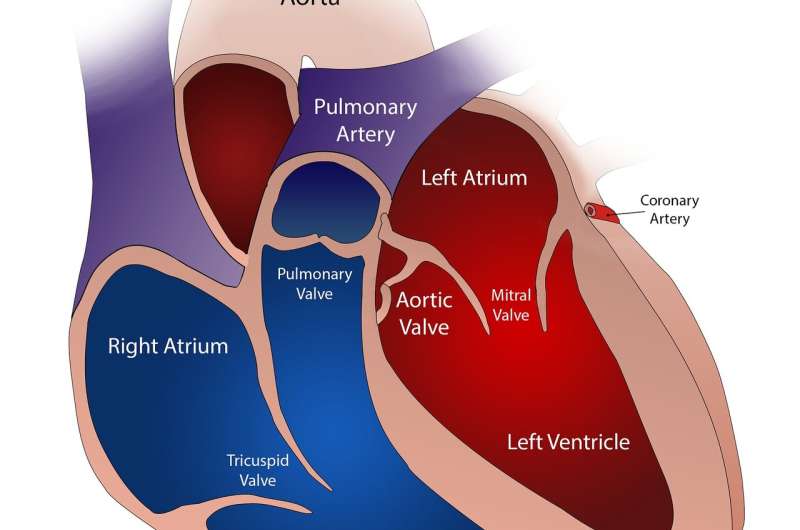
A Cleveland Clinic research team has published an “atlas” of metabolites associated with cardiovascular disease in the European Heart Journal. The novel findings provide key details about the routes and potential branching paths taken by bacteria and metabolic by-products, metabolites.
The study mapped out the multiple by-products of bacteria-processing amino acids associated with cardiovascular disease and then compared that to patient data to assess disease risk in two large cohorts—one in the US and another in Europe.
Bacteria in and on our bodies produce metabolites through processing certain molecules, referred to as precursors. Precursors can come in components of our diet, like protein, or as other metabolized substances. Probiotics (living organisms) and prebiotics (fiber, starch) have increasingly been introduced in foods or supplements as possible clinical interventions.
“The road map generated in the present studies acts as a ‘GPS’ to help guide clinicians and scientists in how to intelligently shift the gut microbiome output into one that makes more beneficial substances,” says Stanley Hazen, MD, Ph.D., a co-author on the study, Chair of the Department of Cardiovascular & Metabolic Sciences and co-section head of Preventive Cardiology at Cleveland Clinic.
Along with confirming additional metabolites associated with cardiovascular risk, researchers developed a method for assessing exactly how much of the metabolites are in the bloodstream. Other methods tend to provide relative levels of metabolite compared to other substances, but not absolute amounts—an advance that allows identification of cutoff values for low versus high risk.
This research, including the absolute amounts of metabolites, helps fight any unintended consequences of altering the microbiome, says Ina Nemet, Ph.D., first author and assistant staff in Cardiovascular & Metabolic Sciences. To shift what metabolites are in our bloodstream in the hopes of preventing a certain condition, we also need to understand what precursors lead to bacteria producing those metabolites.
“If microbes do not make phenylacetic acid for example, they will start making something else,” Dr. Nemet says. “Whether these alternative metabolites are associated with benign or harmful outcomes is critical information for informed therapeutics development.”
The study started with a metabolite called phenylacetylglutamine (PAG), which the research team had previously discovered was linked with elevated chance of events like heart attack or stroke.
PAG is produced from phenylalanine, an amino acid found in many foods, including plant and animal-based proteins. The study also studied gut microbial metabolism of tyrosine and tryptophan, amino acids that share some common metabolic pathways with phenylalanine, to get a “big picture” look, Dr. Nemet says.
“In addition to PAG, microbes can generate a plethora of other metabolites starting from the same precursor,” she says. “This inspired us to investigate these pathways to get a more comprehensive picture on how those metabolites interconnect and are associated with cardiovascular disease.”
More information:
Ina Nemet et al, Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality, European Heart Journal (2023). DOI: 10.1093/eurheartj/ehad333
Journal information:
European Heart Journal
Source: Read Full Article