On Sunday, federal Chief Medical Officer Professor Paul Kelly said most Australians will be offered a vaccine from Oxford-AstraZeneca.
Australia currently has agreements in place to receive 53.8 million doses of the AstraZeneca shot, and 10 million doses from Pfizer-BioNTech.
So how do these two vaccines compare, how will they be used in Australia, and what can we learn from other vaccines?
Comparing the two
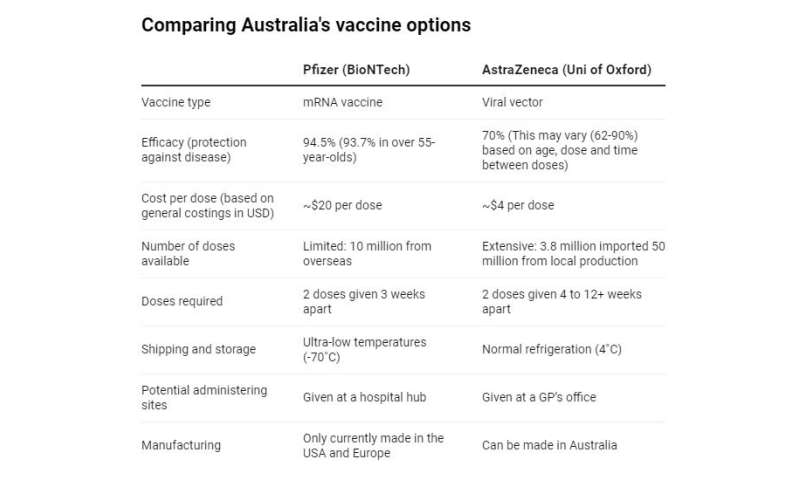
Both the Pfizer and AstraZeneca vaccines induce immunity but in different ways. They both deliver the instructions for how to make a target on the virus for our immune system to recognize the spike protein.
The Pfizer vaccine packages the instructions up in a droplet of fat, while the AstraZeneca vaccine packages the instructions up in the shell of a virus, the adenovirus.
Clinical trials for both vaccines have shown they’re broadly safe.
In terms of efficacy, the Pfizer vaccine protects 94.5% of people from developing COVID.
The AstraZeneca shot protects 70% of people on average—still pretty good and on par with the protection given by a flu vaccine in a good year.
However, the optimal dose and timing of AstraZeneca’s shots is still unclear. One trial reported 62% efficacy, and another 90%, with a low dose for the first shot and/or longer break between doses possibly improving protection. More studies are underway to define this and the Therapeutic Goods Administration, Australia’s regulatory body, will evaluate new data as it comes through.
In any scenario, the AstraZeneca vaccine will still protect the majority of people that receive the vaccine from disease.
While the Pfizer vaccine was more protective in clinical trials, the AstraZeneca vaccine has other advantages that could make it more appropriate for use outside of clinical trials:
we can make the vaccine here in Australia, so we’re not dependent on a supply-chain from overseas
we can ship and store it easily at normal refrigeration temperatures, while the Pfizer vaccine requires temperatures below -70˚C
we can administer it more easily, potentially in GP offices.
From a logistical perspective, the AstraZeneca vaccine has a major advantage. The ability to distribute vaccines can be almost as important as the vaccine’s effectiveness.
The effect of these advantages on the impact of this vaccine shouldn’t be underestimated. We have lots of people to vaccinate, a low disease burden currently, are far away from the rest of the world in terms of shipping, and Australia is a pretty big country, so distribution to rural and remote communities is a massive hurdle.
Efficacy isn’t the only thing we should consider
It can be helpful to look at the flu vaccine as a contrast. The flu vaccine is far from perfect—it provides moderate protection, with effectiveness varying between different groups of people and from season to season. For example, in the 2015/16 season in the United States, the quadrivalent influenza vaccine (which covers four strains) was about 54% effective against laboratory-confirmed influenza.
People know it’s not perfect, but people don’t generally judge whether they’ll receive a vaccine based on its effectiveness alone. We know from talking to the community that many factors influence motivation, especially perceived risk and severity of infection, and confidence in the safety of the vaccine.
Every year, access to flu vaccines is prioritized to those at most risk, such as people with medical conditions, Aboriginal and Torres Strait Islanders and those aged 65 years and older. The public has confidence in this approach. We need to protect those most at-risk first, and we don’t have an issue doing this day-to-day. We now have a similar challenge with the new COVID vaccines.
The best approach for protecting everyone’s health amid the pandemic is to provide different vaccines to different people according to need and availability, at least in the short term. The best vaccine is always the one you can get to the communities that need it before they urgently need it.
Australia’s combination strategy
Because Australia is essentially COVID-free at present, it means we’re in a unique situation that permits a “combination” vaccine strategy.
The Pfizer vaccine is perfect for preventing the most extreme outcomes for people at very high risk of infection or disease: people on the frontlines of the fight against COVID and older people or people with high-risk health conditions.
The AstraZeneca vaccine has the ability to protect a large number of people against disease quickly, because we can make it easily and distribute it quickly.
As a result, Pfizer is likely to be prioritized for people with higher risk and AstraZeneca is likely to be prioritized for everyone else.
We won’t all be able to get the Pfizer vaccine straight away, so for many of us the choice in the short term will be between a 70% efficacious vaccine or no vaccine.
We all stand to benefit from a strategy that protects extremely vulnerable groups from severe disease and aims to rapidly generate immunity in the rest of our community.
There may also be other vaccines that become available. Australia is part of COVAX which can distribute a variety of vaccines, and it also has an agreement for a vaccine made by Novavax, pending the outcome of phase 3 clinical trials. There could be other vaccines that emerge or other agreements developed, and Australia’s strategy will no doubt respond to that.
Nevertheless, both the Pfizer and AstraZeneca vaccines are essential tools in our public health toolkit, with vital roles to play in protecting the entire Australian population. We’ll also need to continue to use other public health tools like testing and contact tracing.
Factoring in effectiveness, availability and distribution challenges, a strategy that uses a combination of the two vaccines for Australia is the best of both worlds.
Source: Read Full Article






