A new study links a gene concentrated in the brain’s cleanup cells, known as microglia, to the inflammation that has increasingly emerged as a key mechanism contributing to Alzheimer’s disease. The findings may offer a new potential target for therapies for the intractable condition.
The gene, known as inositol polyphosphate-5-phosphatase D (INPP5D), is the subject of a collaborative study conducted by researchers from the Icahn School of Medicine at Mount Sinai and the Grossman School of Medicine at NYU Langone Health that appears in the November 30 issue of Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
Microglia are immune cells in the brain that act as scavengers to remove dying cells and amyloid plaques that are associated with the dementia of Alzheimer’s disease. Human genetics studies initially linked INPP5D to the risk for Alzheimer’s disease. Other studies revealed elevated levels of INPP5D in the postmortem brain tissue of Alzheimer’s disease patients, but the specific role(s) that the gene plays in both early or late disease and the mechanism contributing to these altered functions remains unknown.
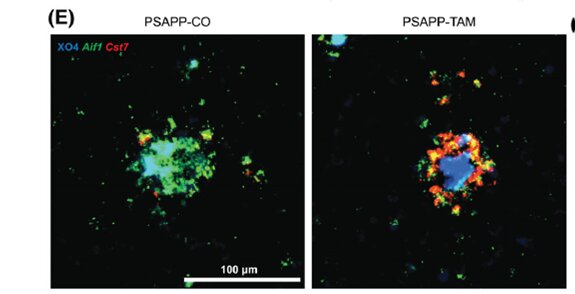
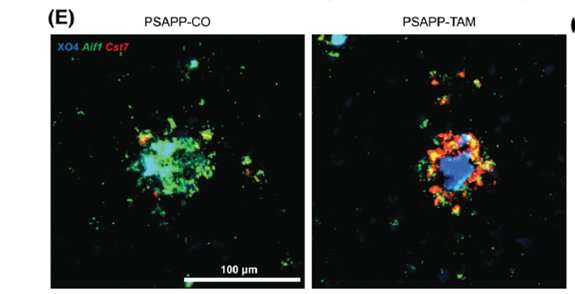
Because INPP5D in the brain is concentrated in microglia, co-senior author Michelle E. Ehrlich, M.D., Professor of Neurology, Pediatrics, and Genetics and Genomic Sciences at Icahn Mount Sinai, used mice genetically engineered to “knock down” (turn off) the mouse INPP5D gene in their microglia at the onset of pathology. This process allowed them to better see the specific impact of the missing gene on brain tissue. They then measured plaque buildup and microglial behavior approximately three months later. Since INPP5D was known to be elevated in the brains of Alzheimer’s patients, the scientists expected that the mice with that gene inactivated would be protected from the amyloid plaques that are hallmarks of Alzheimer’s disease pathology.
“When I looked through the microscope, I was quite surprised to see that the mice lacking INPP5D in their microglia had more plaques that mice with normal microglia,” said Emilie Castranio, Ph.D., a postdoctoral fellow in Dr. Ehrlich’s lab and co-first author on the new paper. “Microglia frequently sit on the edges of the plaques but when INPP5D was knocked down, the plaques were completely covered with them.”
“We are encountering unexpected results more and more with modulation of inflammation genes in Alzheimer’s,” said Dr. Ehrlich. “At this point in our understanding, we still do not know which of these genes to target for therapeutic intervention in humans, or whether to turn them up or turn them off depending on disease stage. Because these experiments are not possible in living humans, we rely on mouse models to show us the way. We also use these mice to help us predict whether a particular gene is more related to disease onset or disease progression, with the caveat that mouse and human microglia differ in important ways. Despite these differences, the plaque-associated gene signature we identified overlaps with human Alzheimer’s disease gene networks.”
When it became clear that the INPP5D knockdown moved microglia around the brain in unexpected ways, Dr. Ehrlich recognized that detailed spatial and quantitative gene expression information was required. Spatial transcriptomics is a molecular profiling method that allows scientists to measure all the gene expression in a tissue sample and map where the expression is occurring. Drs. Ehrlich and Castranio turned to Shane Liddelow, Ph.D., Assistant Professor of Neuroscience, Physiology, and Ophthalmology at NYU Langone, and co-senior author of the new study, who is a world leader in this approach.
Source: Read Full Article