Epithelial ovarian cancer (EOC) exhibits strong dependency on the tricarboxylic acid (TCA) cycle and oxidative phosphorylation to fuel anabolic process. S-palmitoylation refers to the addition of the saturated 16-carbon fatty acid palmitate at a cysteine residue in protein to form a liable thioester bond. The S-palmitoylation modification alters the hydrophobic property of target proteins, regulating their cellular localization, trafficking, stability, activity, and signaling.
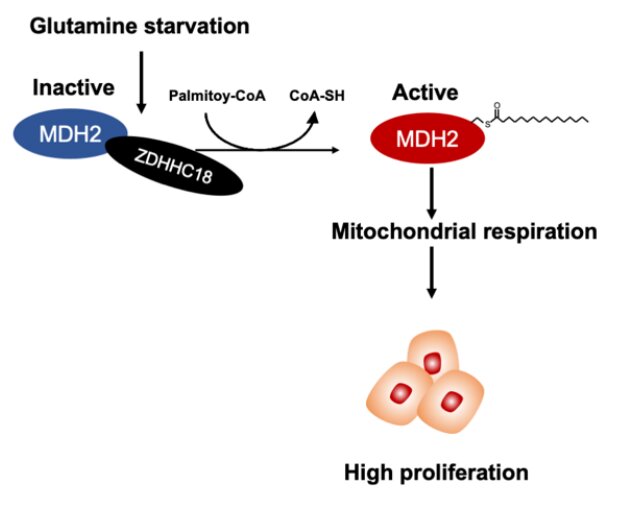
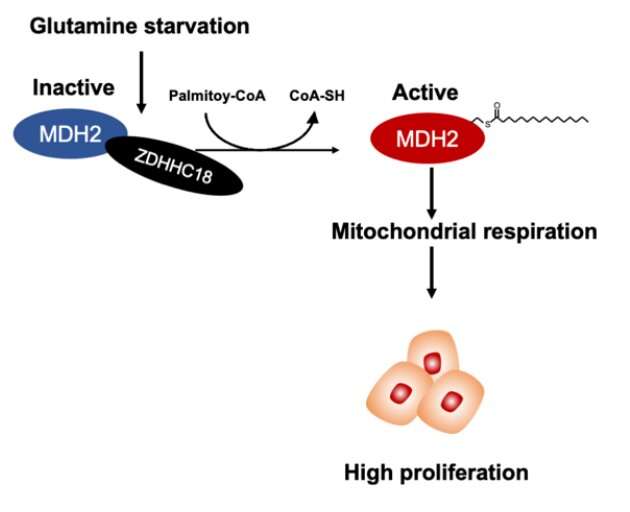
A paper published in Science China-Life Sciences shows that malate dehydrogenase 2 (MDH2), a key enzyme of the TCA cycle, is palmitoylated at cysteine 138 (C138) residue, resulting in increased activity of MDH2. The paper shows that ZDHHC18 acts as a palmitoyltransferase of MDH2. Glutamine deprivation enhances MDH2 palmitoylation by increasing the binding between ZDHHC18 and MDH2. MDH2 silencing represses mitochondrial respiration as well as ovarian cancer cell proliferation both in vitro and in vivo. Intriguingly, re-expression of wild-type MDH2, but not its palmitoylation-deficient C138S mutant, sustains mitochondrial respiration and restores the growth as well as clonogenic capability of ovarian cancer cells. Notably, MDH2 palmitoylation level is elevated in clinical cancer samples from patients with high-grade serous ovarian cancer.
These observations suggest that MDH2 palmitoylation catalyzed by ZDHHC18 sustains mitochondrial respiration and promotes the malignancy of ovarian cancer, yielding possibilities of targeting ZDHHC18-mediated MDH2 palmitoylation in the treatment of EOC.
Source: Read Full Article