Scientists believe the most important tool to contain the ongoing coronavirus disease 2019 (COVID-19) pandemic is rapid vaccination. The pandemic has been caused by the rapid transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Numerous studies have been conducted to assess the effectiveness of existing COVID-19 vaccines in the general population. Some of these studies have reported the high effectiveness of the BNT162b2 vaccine developed by Pfizer-BioNTech and the ChAdOx1 nCoV-19 vaccine by Oxford-AstraZeneca, against the Alpha (B.1.1.7) and preceding variants.
Owing to mutations, there has been a continuous emergence of SARS-CoV-2 variants, which have threatened the effectiveness of the vaccines. In vitro studies have indicated a reduced neutralization activity of vaccine-induced antibodies against some of the SARS-CoV-2 variants, e.g., Delta variant (B.1.617.2). This variant has caused a surge in the number of COVID-19 cases in many countries across the world, including those with high vaccination coverage, such as the UK. Due to the high infectiousness of the Delta variant, it has been classified as a Variant of Concern (VoC).
Vaccine Effectiveness and the Delta Variant
Scientists have pointed out the scarcity of real-time evidence on the effectiveness of the available vaccines against the Delta variant. A recent study that used data from the English symptomatic testing program revealed that the efficacy of a single dose of BNT162b2 or ChAdOx1 vaccine was much lower against symptomatic infection with the Delta variant than the Alpha variant. However, both the vaccines were found to be effective against the Delta variant, with a minor reduction in efficacy, after completion of the two-dose regime.
Another study conducted in Scotland indicated reduced effectiveness of vaccines against the Delta variant, even after administering two doses of the vaccines. This study also showed the high efficacy of the vaccines against the Alpha variant. The impact of vaccination on hospital admission was not reported.
A New Study
Now, a new study published on the medRxiv* preprint server focused on determining the effectiveness of the BNT162b2, ChAdOx1, and mRNA-1273 vaccines by conducting SARS-CoV-2 PCR-positive tests. This study used an extensive community-based survey (Office for National Statistics COVID-19 Infection Survey) of individuals living in randomly selected private households across the UK. Irrespective of symptoms, vaccination, and prior infections, RT-PCR tests were conducted in these households on a pre-determined schedule.
Researchers determined the effectiveness of the vaccines based on overall RT-PCR positivity, self-reported symptoms, and the cycle threshold (Ct) value that indicated the viral load. This study was conducted in two phases, and the first phase was between 1 December 2020 (commencement of vaccination program) and 16 May 2021, during which period the Alpha variant was dominant.
The second phase was from 17 May 2021 to 1 August 2021, when the Delta variant was dominant. Researchers also determined the variation in vaccine effectiveness by long-term health conditions among two age groups, i.e., 18-34 years and 35-65 years. Also, vaccine efficacy was assessed based on the interval between first and second vaccination and prior infection. Finally, the viral burden, using Ct values, was assessed among newly infected individuals who became PCR-positive after 14 days of receiving the second vaccine dose.
Main Findings
This study reported that the effectiveness of the BNT162b2 and ChAd0x1 vaccines against the Delta variant was reduced compared to other SARS-CoV-2 variants, such as the Alpha strain. Researchers found that a single dose of the mRNA-1273 vaccine had similar or better effectiveness than a single dose of the BNT162b2 or ChAdOx1 vaccine. The effectiveness of two doses was comparable to the immune protection triggered after natural SARS-CoV-2 infection.
The degree of immune responses induced by the BNT162b2 and ChAdOx1 vaccines differed significantly. Both the vaccines were found to be highly effective against the new PCR positives, but a decline in protection was observed when the viral load was high, especially, in the case of the BNT162b2 vaccine.
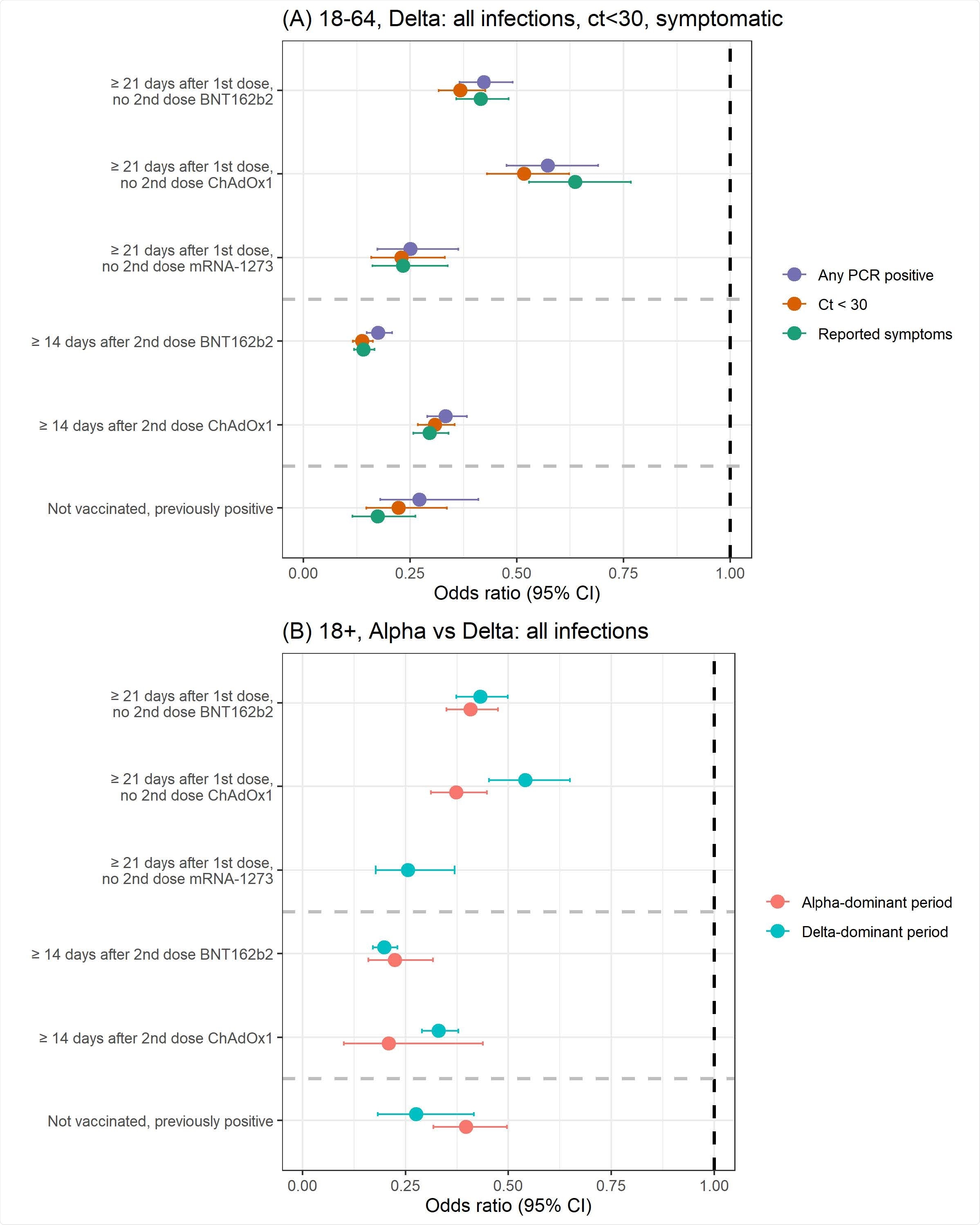
In the context of vaccine effectiveness with varied dose intervals, researchers found no significant variation in vaccine effectiveness. However, COVID-19 convalescent individuals who were vaccinated were found to possess greater immune protection compared to vaccinated individuals without a history of SARS-CoV-2 infection.
Surprisingly, individuals who completed the two doses of vaccine regime and were infected with the Delta variant exhibited a viral load comparable to those who were not vaccinated and were infected with this strain.
Conclusion
The main strength of this study is its sample size and design. Additionally, researchers accounted for the risk factors that influence vaccination. Some of the factors considered in this study are commonly documented in electronic health records, e.g., the long-term health conditions of a patient. However, most of the studies solely rely on the data available in the electronic databases, which may miss small intrinsic details. The current study incorporated data beyond what is available in these databases. One of the limitations of this study is that even though it has included numerous potential confounders, it might have excluded unknown confounders or misclassified a prior infection status. This might bring about a biased result.
The authors of this study stated that even though SARS-CoV-2 vaccination reduces new infections, the effectiveness in lowering the viral load against the Delta strain was found to be reduced.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Pouwels, B.K. et al. (2021) Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv 2021.08.18.21262237; doi: https://doi.org/10.1101/2021.08.18.21262237, https://www.medrxiv.org/content/10.1101/2021.08.18.21262237v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Coronavirus, Coronavirus Disease COVID-19, CT, Efficacy, Hospital, in vitro, Pandemic, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article






