Nanoparticles may be created using several methods. Some of them may occur in nature as well. The methods of creation include attrition and pyrolysis. While some methods are bottoms up, some are called top down. Top down methods involve braking the larger materials into nanoparticles.
Nanoparticle Synthesis |
|
| Top-Down via | Bottom-Up via |
| Attrition / Milling | Pyrolysis |
| Inert gas condensation | |
| Solvothermal reaction | |
| Sol-Gel fabrication | |
| Structured media |
Attrition
Attrition methods include methods by which macro or micro scale particles are ground in a ball mill, a planetary ball mill, or other size reducing mechanism. The resulting particles are air classified to recover nanoparticles.
- Involves mechanical thermal cycles
- Yields
- broad size distribution (10-1000 nm)
- varied particle shape or geometry
- impurities
- Application
- Nanocomposites
- Nano-grained bulk materials
Bottoms up methods
These are further classified according to phases:
- Gas (Vapor) Phase Fabrication: Pyrolysis, Inert Gas Condensation
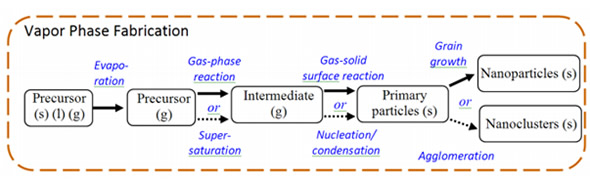
- Liquid Phase Fabrication: Solvothermal Reaction, Sol-gel, Micellar Structured Media
Pyrolysis
In pyrolysis, a vaporous precursor (liquid or gas) is forced through a hole or opening at high pressure and burned. The resulting solid is air classified to recover oxide particles from by-product gases. Pyrolysis often results in aggregates and agglomerates rather than singleton primary particles.
Instead of gas, thermal plasma can also deliver the energy necessary to cause evaporation of small micrometer size particles. The thermal plasma temperatures are in the order of 10,000 K, so that solid powder easily evaporates. Nanoparticles are formed upon cooling while exiting the plasma region. Examples of plasma used include dc plasma jet, dc arc plasma and radio frequency (RF) induction plasmas.
For example, silica sand can be vaporized with an arc plasma at atmospheric pressure. The resulting mixture of plasma gas and silica vapour can be rapidly cooled by quenching with oxygen, thus ensuring the quality of the fumed silica produced.
The advantages of vapor phase pyrolysis include it being a simple process, cost effective, a continuous operation with high yield.
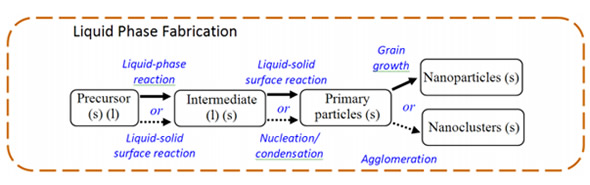
Liquid phase synthesis methods
The liquid phase fabrication entails a wet chemistry route.
Methods are:
- Solvothermal Methods (e.g. hydrothermal)
- Sol-Gel Methods
- Synthesis in Structure Media (e.g., microemulsion)
Effectiveness of Solvothermal Methods and Sol-gel methods demands a simple process, low cost, continuous operation and high yield.
Solvothermal process
Precursors are dissolved in hot solvents (e.g., n-butyl alcohol) and solvent other than water can provide milder and friendlier reaction conditions If the solvent is water then the process is referred to as hydrothermal method.
Sol-gel process
The sol-gel process is a wet-chemical technique (also known as chemical solution deposition) widely used recently in the fields of materials science and ceramic engineering.
Steps include:
- Formation of stable sol solution
- Gelation via a polycondensation or polyesterification reaction
- Gel aging into a solid mass. This causes contraction of the gel network, also phase transformations and Ostwald ripening.
- Drying of the gel to remove liquid phases. This can lead to fundamental changes in the structure of the gel.
- Dehydration at temperatures as high as 8000 degree C, used to remove M-OH groups for stabilizing the gel, i.e., to protect it from rehydration.
- Densification and decomposition of the gels at high temperatures (T > 8000 degree C), i.e., to collapse the pores in the gel network and to drive out remaining organic contaminants
The ultimate microstructure of the final component will clearly be strongly influenced by changes implemented during this phase of processing. The precursor sol can be either deposited on a substrate to form a film (e.g. by dip-coating or spin-coating), cast into a suitable container with the desired shape (e.g. to obtain a monolithic ceramics, glasses, fibers, membranes, aerogels), or used to synthesize powders (e.g. microspheres, nanospheres).
Advantages of the sol-gel process
Advantages of the sol-gel process are that it is a cheap and low-temperature technique that allows for the fine control of the product’s chemical composition. Even small quantities of dopants, such as organic dyes and rare earth metals, can be introduced in the sol and end up uniformly dispersed in the final product.
Sources
- courses.washington.edu/…/Lecture4-Overney-NP-Synthesis.pdf
- dspace.cc.tut.fi/…/keskinen.pdf?sequence=1
- www.ieni.mi.cnr.it/…/nanoparticle-synthesis-and-characterization
- www.nanobiotec.iqm.unicamp.br/…/…n%20nanoparticles-chapter%205.pdf
- neutrons.ornl.gov/…/050616_goodman_wayne_nni05.pdf
- documents.irevues.inist.fr/bitstream/handle/2042/6269/1020.pdf?…
- http://arxiv.org/ftp/arxiv/papers/0801/0801.3280.pdf
Further Reading
- All Nanoparticle Content
- Nanoparticles – What are Nanoparticles?
- Nanoparticle Uniformity
- Properties of Nanoparticles
- Nanoparticle Colloids
Last Updated: Apr 3, 2019

Written by
Dr. Ananya Mandal
Dr. Ananya Mandal is a doctor by profession, lecturer by vocation and a medical writer by passion. She specialized in Clinical Pharmacology after her bachelor's (MBBS). For her, health communication is not just writing complicated reviews for professionals but making medical knowledge understandable and available to the general public as well.
Source: Read Full Article






