Researchers from the Case Comprehensive Cancer Center uncovered the potentially important role of the protein-coding gene, MYO10, in tumor development and immune therapy response. Their findings were published today in Science Advances.
The team, led by Youwei Zhang, an associate professor of pharmacology at the Case Western Reserve University School of Medicine, found breast tumors with high levels of MYO10—including the most malignant triple-negative breast cancers—responded favorably to immune checkpoint blockade (ICB) therapy, supporting the use of this therapy to treat these types of tumors.
ICB is an innovative treatment that uses medications known as immune checkpoint inhibitors—medications that can help the body’s immune system recognize and attack cancerous cells.
The team’s findings also suggest that the use of ICBs should be avoided in breast tumors with low MYO10 because these tumors actually grew larger after ICB therapy.
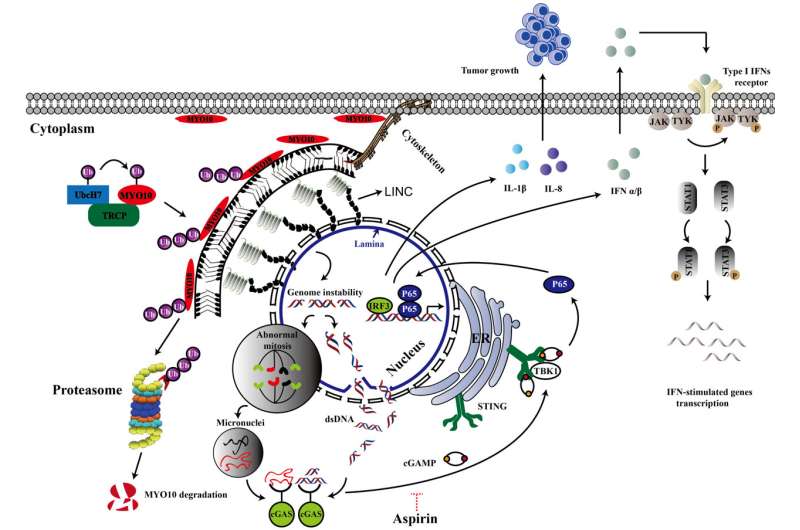
Studies found that high levels of MYO10 induce chronic inflammation in tumors, which reduces the ability of key immune cells—known as T cells—to slow tumor growth.
At the same time, however, this inflammatory environment in the tumor increased the ability of immune cells to provoke an immune response. This eventually allowed tumor cells to respond to ICB therapy, improving outcomes.
Proteins called interferon and interleukin—and other factors that regulate their production—are part of the body’s natural defenses. When they sense germs or cancer cells in the body, they boost the immune system, triggering killer immune cells to fight these invaders.
In this study, the researchers found that high levels of MYO10 increased the presence of interferons; conversely, low levels of MYO10 correlated with reduced production of interferons. These findings at least partly explain why MYO10-low tumors failed to respond to ICB therapy, the researchers explained.
Consistent with a chronic inflammation environment in tumors with high levels of MYO10 that led to loss of T cell function, treatment with aspirin to reduce inflammation significantly reduced the growth of breast tumors overexpressing MYO10.
This is consistent with studies showing that long-term use of aspirin or nonsteroidal anti-inflammatory drugs significantly reduced tumor risk for esophageal, colorectal and stomach cancers, as well as—to a smaller degree—in breast, lung and prostate cancers.
The team members further uncovered how MYO10 drives tumor growth. They found that MYO10 regulates the genome stability and cancer growth through mediating the nucleus shape. They also discovered that the protein level of MYO10 is critical for this function.
They went on to show how the protein recycling system tightly controls the expression level of MYO10. These findings support the tumor studies, highlighting the importance of MYO10 in tumor development and immune therapy response.
“This is the first study to link MYO10 with genomic instability and to validate the role of MYO10 in tumor development and immune therapy response,” said Zhang, a member of the Molecular Oncology Program of the Case Comprehensive Cancer Center. “We will continue these studies and are eager to see how this knowledge can have a clinical impact on patient care. In addition, we hope that these findings will lead to more effective therapy options for breast cancer patients and the elimination of less effective treatments.”
Source: Read Full Article






