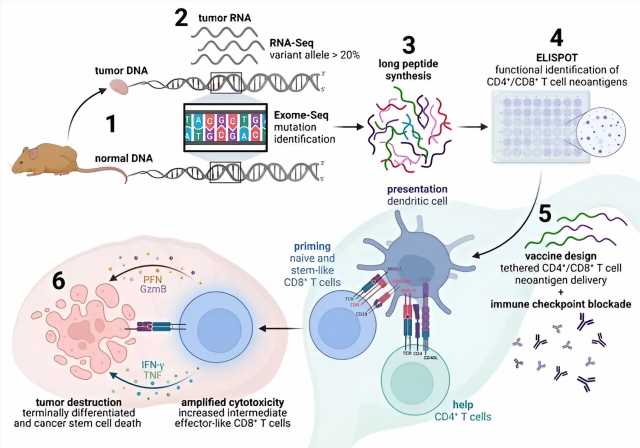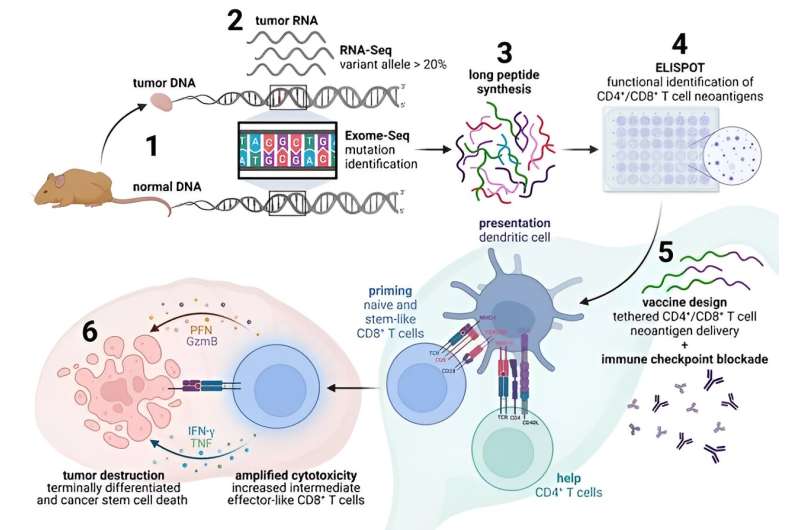
Scientists are on the hunt for a unique set of mutations, called “neoantigens,” that let the immune system distinguish tumor cells from normal cells. Their goal is to help the immune system react to neoantigens and target tumor cells for destruction.
This area of research has led to life-saving antibody therapeutics, such as immune checkpoint inhibitors, which rely on antibodies to help immune cells kill tumors. Unfortunately, antibody-based cancer immunotherapies don’t work for all patients.
At La Jolla Institute for Immunology (LJI), Professor Stephen Schoenberger, Ph.D., and his colleagues are looking beyond antibodies. Schoenberger’s lab leads pioneering research into how the immune system’s CD4+ “helper” T cells detect neoantigens.
Now Schoenberger and his colleagues have published a pair of studies that show how we might harness CD4+ T cells while boosting the cancer-fighting power of CD8+ “killer” T cells. In fact, the researchers demonstrate a new kind of vaccine design that recruits both types of T cells to destroy large tumors.
“Therapeutic cancer vaccines can work,” says Schoenberger, who serves as a member of the LJI Center for Cancer Immunotherapy. “But they should leverage the natural synergy of CD4+ and CD8+ T cells.”
Researchers help CD4+ T cells detect tumors
As Schoenberger points out, CD4+ and CD8+ T cells already work together when fighting viruses and bacteria. “Why not learn from the immune system’s natural way of keeping us protected and turn that against cancer?” he says.
In a paper published recently in Nature Immunology, Schoenberger worked closely with LJI Professor Bjoern Peters, Ph.D,. to demonstrate the essential role of CD4+ T cells in recognizing tumor cells. Their strategy depends on an innovative way to predict which tumor neoantigens will spark a strong CD4+ T cell response.
As Schoenberger explains, tumor cells arise from normal cells in the body. This means the body has a harder time recognizing tumor cells as dangerous. Other threats, such as viruses, tend to carry around very un-human looking peptide sequences. “With prompting from CD4+ T cells, immune cells called dendritic cells can capture these peptide sequences and show them to CD8+ T cells—sending the immune system into red alert. “CD8+ T cells execute the tumor,” says Schoenberger, “but they require the cooperation of CD4+ T cells to do so efficiently.”
But tumor cells share most of their peptide sequences with normal cells, and are therefore harder for the immune system to “see.” To get around this problem, Schoenberger and Peters have devised computational tools to identify the genetic mutations and specific peptides that serve as neoantigens to distinguish tumor cells from their neighbors.
The Nature Immunology study shows that CD4+ T cells that recognize a single target mutation can drive a diverse CD8+ T cell response that eradicates large established tumors . The researchers tested T cells recognizing this target mutation for “avidity,” which is how strongly their antigen receptors bind to the neoantigen. Their surprising results showed that neoantigen-specific CD4+ T cells can mediate their effect across a range of affinities.
“This is brand new because no one has ever studied the neoantigen-specific CD4+ repertoire at the level of T cell receptors,” says Schoenberger.
The researchers also found that the most effective responses happened when the transferred CD4+ T cells were induced to develop into stem cell memory-like CD4+ T cells. This type of T cell are endowed with special properties of longevity and the ability to generate powerful effector cells. As Schoenberger’s research spans the lab to the clinic, these findings will be translated to clinical trials in the near future.
New vaccine brings T cells together
In a second study, published recently in the Journal of Clinical Investigation, Schoenberger and his colleagues showed how a new vaccine strategy can induce CD4+ T cells and CD8+ T cells to work together to destroy large, aggressive tumors in a mouse model.
For the study, Schoenberger collaborated with Joseph Dolina, Ph.D., a senior scientist at Pfizer Inc., and former member of the Schoenberger Lab (Pfizer has no financial disclosures to this specific study).
The team began with an aggressive squamous cell tumor that contained a low number of mutations, as many human cancers do. The researchers identified 270 mutations that make this tumor stand out from normal cells, and they performed in-depth studies on 39 of these mutations.
They narrowed that group down to five mutations that were recognized by the natural anti-tumor T cell response—with some mutations targeted by CD4+ T cells and others by CD8+ T cells. Remarkably, only mutations targeted by both CD4+ and CD8+ T cells were capable of triggering protective or therapeutic responses against the tumor.
“These neoantigens had to be physically linked to mediate therapy,” says Schoenberger. “We could make large tumors go away so long as the vaccine activated both CD4+ and CD8+ T cells via the same antigen-presenting cell.”
Going forward, Schoenberger plans to work with his clinical colleagues at the UC San Diego Moores Cancer Center to study whether this type of linked vaccine is effective in human patients. He hopes a future clinical trial can give hope to patients with especially aggressive tumors.
“The other message here is that we think we can greatly increase the number of patients who could benefit from checkpoint blockade immunotherapy if we combine it with a personalized cancer vaccine,” says Schoenberger.
More information:
Spencer E. Brightman et al, Neoantigen-specific stem cell memory-like CD4+ T cells mediate CD8+ T cell-dependent immunotherapy of MHC class II-negative solid tumors, Nature Immunology (2023). DOI: 10.1038/s41590-023-01543-9
Joseph S. Dolina et al, Linked CD4+/CD8+ T cell neoantigen vaccination overcomes immune checkpoint blockade resistance and enables tumor regression, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI164258
Journal information:
Journal of Clinical Investigation
,
Nature Immunology
Source: Read Full Article