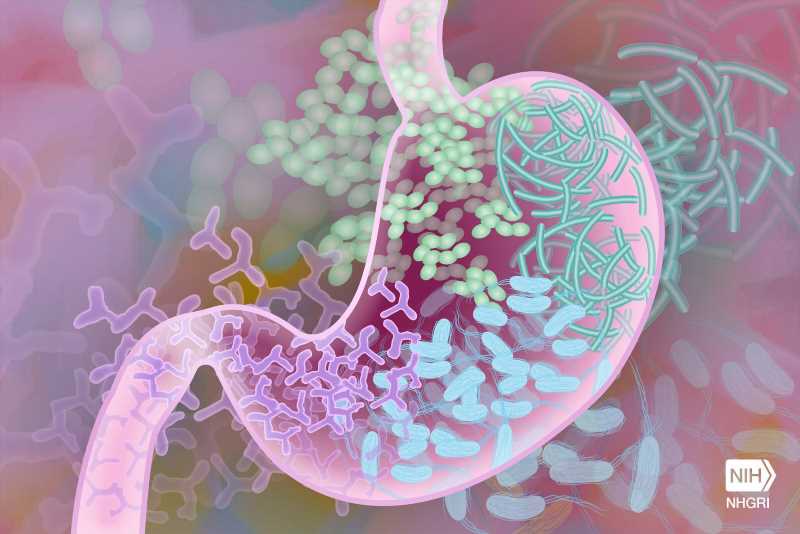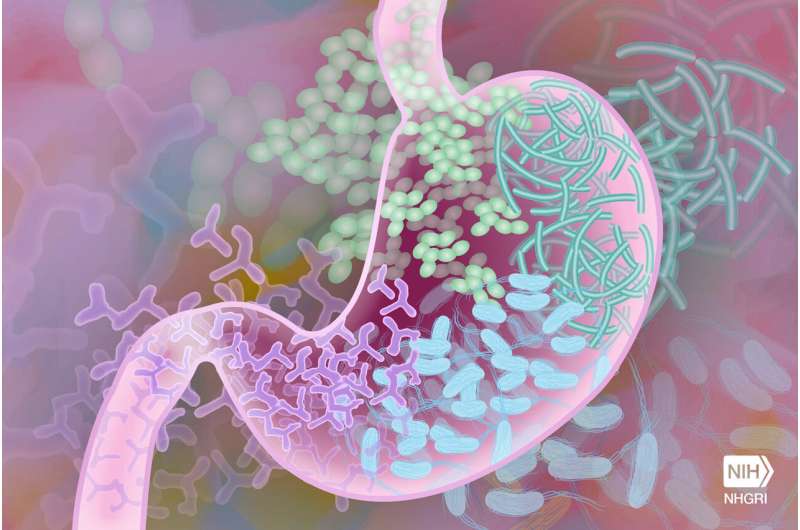
Trust your gut? Your doctor likely will, too. Probiotics engineered to sense and report signs of bowel inflammation could deliver firsthand knowledge of your system’s inner workings—provided they are not killed or dispersed in the process.
Working toward making this scenario a clinical reality, Rice University bioengineers developed a platform that allows engineered biosensor bacteria safe passage through the gastrointestinal (GI) tract in an animal model, according to a study published in Biomaterials. “Good” bacteria designed to produce a fluorescent protein in response to physiological signs of disease fared and performed well inside the rat gut, protected inside alginate particles.
By combining synthetic biology with creative biomaterial design, the research groups of Rice faculty members Jeffrey Tabor and Omid Veiseh designed a platform that could eventually be deployed in a clinical setting not only to diagnose inflammatory bowel disease (IBD), but also to monitor disease progression, assess treatment response and help provide care tailored to individual patients’ unique gut microbiome.
“For our proof-of-concept study, we chose inflammatory bowel disease, an autoimmune disorder that causes painful and recurrent inflammation flares,” said Elena Musteata, a graduate student in systems, synthetic and physical biology in the Tabor lab. “But gut health plays many important roles in the human body, affecting metabolism, immunity, brain function and other systems. As we discover more biomarkers for different diseases, we can use this platform to diagnose and monitor a lot of different health conditions.”
The platform could help replace what is often a prolonged and complex diagnostic process for IBD—which involves invasive, time-consuming procedures like colonoscopies and biopsies and relies, in part, on subjective self-monitoring—with a much simpler and faster procedure.
“With our system, patients could theoretically receive a prescription for the capsules and simply drop off a stool sample after ingestion, eliminating the need for repeated colonoscopies or biopsies,” said Samira Aghlara-Fotovat, a bioengineering graduate student in the Veiseh lab. “Monitoring disease progression over time or keeping track of how a patient is responding to a given therapy could be much more accessible using a platform like this.”
According to Musteata, a quicker diagnosis could lead to better patient outcomes: “Especially with IBD, it’s very important to minimize the delay between symptom onset and treatment,” she said. “Having a way to assess gut health within a short amount of time and then take action could really generate a significant advance in the clinical management of chronic inflammation and other gut-related disorders.”
Another reason the platform may lead to improved patient outcomes is the ability to tailor treatments to individual patients’ physiology.
“If, in the future, we encapsulate a diverse range of biosensor strains, we could get an idea about a specific person’s inflammatory profile and then use that information to develop a more personalized diagnosis and treatment,” Aghlara-Fotovat said.
While it’s not new for bacteria to be genetically programmed to detect disease biomarkers in the GI tract, once inside the body, they face challenges surviving harsh conditions like low pH, destructive enzymes and bile salts. Moreover, free bacteria can disperse widely in the gut, making analysis more difficult and potentially less accurate.
“Our study demonstrated that encapsulating biosensor bacteria in protective alginate particles enables their robust survival and diagnostic function in live animals,” Musteata said. “We characterized the effect of encapsulation on the viability, population dynamics and performance of a thiosulfate-sensing bacterial strain previously developed in our lab.”
The engineered strain produces a fluorescent green protein in response to thiosulfate—a compound associated with gut inflammation ⎯ making it possible to assess colon inflammation by measuring bacterial fluorescence after passage through the animal GI tract.
“We found that encapsulation offers several advantages for intestinal biosensing with engineered bacteria,” Musteata said. “First, because capsules are macroscopic, they can be more easily identified and analyzed than free bacteria. Additionally, because bacteria are spatially concentrated within the capsules, their reporter gene expression is easier to visualize than in the same number of free bacteria. Finally, encapsulation can protect bacteria from environmental hazards. For example, we were able to improve the survival of probiotic E. coli under acidic conditions.”
While developing and optimizing the platform was a challenging process, the researchers stressed the necessity of ensuring its reproducibility as a prerequisite for eventual clinical use.
“As we worked on developing our platform, we encapsulated bacteria countless times, optimizing each step of the process along the way. There were so many aspects that needed to be characterized well in order to make a clinically translatable product,” Aghlara-Fotovat said.
“It was really cool to see two exciting technologies come together and expand their existing capabilities,” she added.
Musteata said she values the experience of working “not only to design biosensor bacteria as potential diagnostics, but also to collaborate so closely on interdisciplinary projects that seek to translate these systems into medical applications.”
“Even 10 years ago, this idea of engineered probiotics acting as small diagnostic robots that could travel inside your body and detect your disease state was largely in the realm of science fiction. However, groups at Rice and numerous other institutions are now making real progress toward deploying this kind of technology,” Musteata said.
Tabor is a professor of bioengineering and biosciences. Veiseh is a Rice associate professor of bioengineering and Cancer Prevention and Research Institute of Texas scholar.
More information:
Samira Aghlara-Fotovat et al, Hydrogel-encapsulation to enhance bacterial diagnosis of colon inflammation, Biomaterials (2023). DOI: 10.1016/j.biomaterials.2023.122246
Journal information:
Biomaterials
Source: Read Full Article