
Schools can reopen safely ‘without all of the teachers being vaccinated’: CDC director
CDC Director Dr. Rochelle Walensky tells ‘Fox News Sunday’ returning students to school requires a ‘layered mitigation approach.’ She also discusses the COVID-19 vaccine rollout and new variants of the disease.
The University of Oxford has launched a trial to study the safety and efficacy of the AstraZeneca COVID-19 vaccine in children as young as 6. The study, which Oxford University said is the first of its kind, will involve 300 volunteers, 240 of which will receive the vaccine while the 60 others will receive a meningitis shot.
The university said the 60 who will receive the meningitis shot will serve as the control, and that the jab is safe in children but also is expected to produce similar reactions such a sore arm.
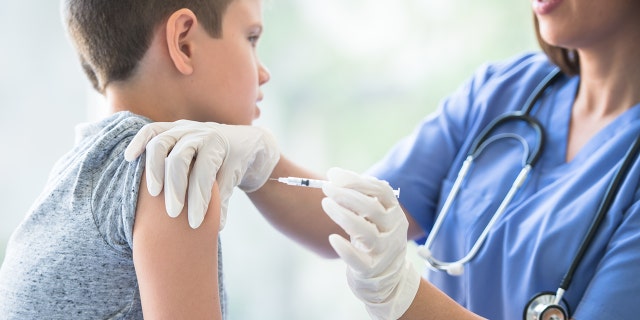
Researchers are aiming to enroll 300 volunteers in the latest trial.
(iStock)
The trial, which is aimed to study the AstraZeneca vaccine in children and teens aged 6 to 17 will build on previous data that showed the jab’s safe and effective when used on adults. Pfizer recently announced that a trial involving children as young as 12 reached full enrollment, and Moderna is planning a trial for children ages 12-17.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, professor of pediatric infection and immunity, and the Oxford University trial’s chief investigator said in a news release.
None of the coronavirus vaccines that have been approved for use have been OK’d for use in children. In the U.S., Pfizer and BioNTech’s vaccine received emergency use authorization for children as young as 16, but Moderna, the second to receive such authorization, was only approved for those over 18.
In the U.S., the American Academy of Pediatrics recently called for everyone 16 and older who meet the criteria for priority vaccination to get one, and renewed its push for more trials involving children and teens.
“It is critical that pediatric patients of all ages be included in trials as quickly as possible,” Dr. Lee Savio Beers, AAP president, said. “We are especially concerned about children who belong to racial, ethnic, and cultural groups that have been disproportionately affected by the pandemic or who have underlying conditions that place them at increased risk for developing severe COVID-19 infection.”
Over 2.9 million children in the U.S. have been diagnosed with COVID-19 since the start of the pandemic, although the number of pediatric cases diagnosed worldwide is unclear.
Source: Read Full Article






