A new research study at the Institute of Molecular Biology and Biotechnology (IMBB) of the Foundation for Research and Technology-Hellas (FORTH), published today in The EMBO Journal, reveals an intricate mechanism that impacts aging by controlling the number and the metabolic activity of mitochondria.
Mitochondria are specialized organelles that mainly function as energy producers inside the cells of the human body, as well as, of other less complex eukaryotic organisms. Mitochondria adjust their function, morphology, and number, in response to the energy demands of cells and the whole organism.
This adaptability is facilitated by complex molecular mechanisms that govern mitochondrial biogenesis, which are not fully understood. Importantly, aberrant mitochondrial biogenesis has been linked to common age-associated diseases and severe genetic syndromes, including neurodegenerative disorders, heart failure, acute kidney injury, and type 2 diabetes.
IMBB researchers Dr. Ioanna Daskalaki, Dr. Maria Markaki and Dr. Elias Gikas, led by Dr. Nektarios Tavernarakis (Professor at the Medical School of the University of Crete, and Chairman of the Board of Directors at FORTH), have discovered that specific proteins, involved in the cytoplasmic mRNA degradation pathway, play a critical role in the regulation of mitochondrial abundance and function, during aging and under conditions of stress.
Mitochondria display unique characteristics, resembling prokaryotes; they have their own genetic material (mitochondrial DNA), but also rely on the DNA in the cell’s nucleus that encodes the vast majority of mitochondrial proteins. While the production of mitochondrial DNA-encoded proteins is well understood, the precise regulation of nuclear DNA-encoded, mitochondrial protein synthesis remains unclear.
Initially, genetic information from the nuclear DNA is transferred to the cytoplasm in the form of mRNA, which carries the required instructions for the generation of mitochondrial proteins. This dedicated protein synthesis process occurs on the outer membrane of mitochondria, and its regulation has been under intense investigation.
“The capability of mitochondria to respond rapidly to external stressors and to effectively adjust their number and function to meet the energy requirements of the cell is truly impressive. Recent findings indicate that nuclear mRNAs encoding mitochondrial proteins are transported and translated near mitochondria, whereas new proteins are immediately imported into these organelles. How is this special localization achieved and how is this process regulated?” Professor Nektarios Tavernarakis said.
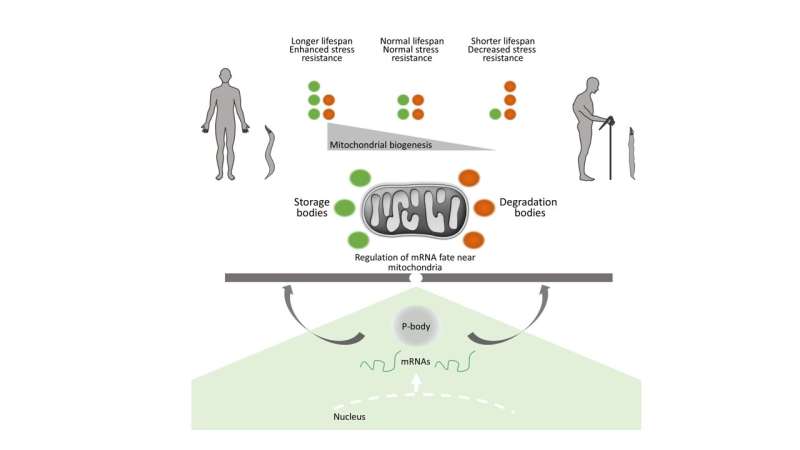
“Given the swiftness of this response, we hypothesized that in order to ensure organismal survival under stress conditions, cells must have evolved sophisticated mechanisms that allow them to mount such rapid responses. Indeed, we identified specific mRNA-binding proteins that form foci near mitochondria, and mediate storage or degrading nuclear mRNAs that encode mitochondrial proteins.”
“These foci control the production of mitochondrial proteins, which regulate oxygen consumption, energy production, and mitochondrial DNA expression. Such mRNA-binding proteins are ubiquitously expressed in all human cells and tissues, and play a critical role in numerous biological processes. Our study uncovered a novel and unanticipated function of these proteins in the regulation of mitochondrial biogenesis and cell survival under stress.”
Using the nematode Caenorhabditis elegans, an experimental animal that offers significant advantages for research on aging, the IMBB researchers demonstrated that the mRNA degradation and deadenylation complexes form distinct foci that associate closely with mitochondria, in somatic cells. The specific localization of these structures in the vicinity of mitochondria allows the regulation of mRNA transcript fate and, consequently, influences mitochondrial biogenesis during aging and under conditions of stress.
Therefore, components of these two macromolecular complexes determine the number and function of mitochondria, modulating life expectancy and stress resistance.
The new findings highlight the importance of mRNA storage and degradation, near mitochondria, as a mechanism for maintaining cellular and organismal homeostasis. “Indeed, disruption of the expression of certain components of mRNA metabolism resulted in premature aging, and the early onset of age-related pathologies, such as reduced motility, and sensitivity to challenging environmental conditions, including diminished tolerance of high temperatures, or exposure to toxic chemical agents.” Professor Tavernarakis commented.
The study published today provides critical insights into the complex mechanisms of aging and enables the development of effective strategies to battle age-related and other severe diseases, aiming to maintain good health and quality of life. Furthermore, these research findings will contribute to the establishment of new biomarkers of aging, and genetic targets for future pre-clinical studies, with the ultimate goal of combating diseases associated with impaired mitochondrial biogenesis and function.
More information:
Ioanna Daskalaki et al, Local coordination of mRNA storage and degradation near mitochondria modulates C. elegans ageing, The EMBO Journal (2023). DOI: 10.15252/embj.2022112446
Journal information:
EMBO Journal
Source: Read Full Article






