Investigators have found that dasatinib, a drug commonly used to treat chronic myeloid leukemia, is strongly associated with kidney injury.
The study team strongly believes this study will impact clinical practice significantly, changing standard of care and possibly introducing new black box warnings for dasatinib.
Furthermore, the researchers point out that the incidence of kidney injury is a previously unknown severe side effect for this drug. This side effect, they report, is advertised to be rare; however, they observed it in 10% of all participants taking dasatinib. Of concern, they say, is that patients taking dasatinib are currently not screened for kidney injury or dysfunction, making them susceptible to chronic kidney disease. The results are published in the Clinical Journal of the American Society of Nephrology.
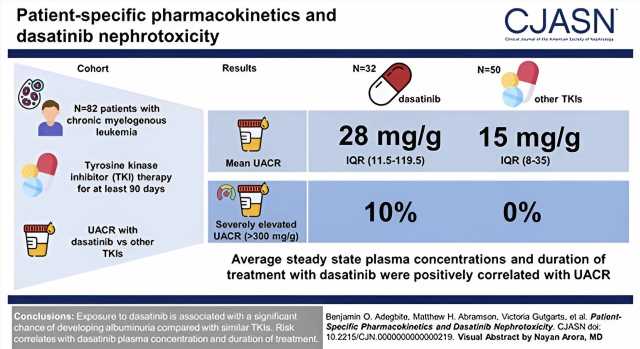
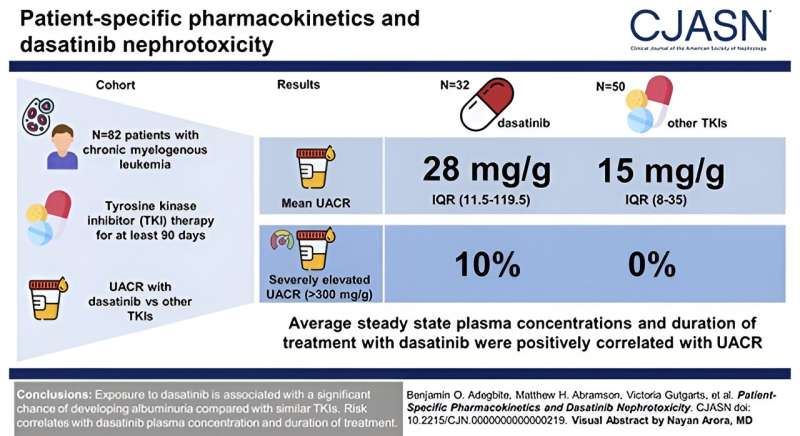
The study included 82 participants who have been on long-term tyrosine-kinase inhibitor treatment for chronic myeloid leukemia; 32 participants were treated with dasatinib and 50 were treated with other, similar drugs.
Significantly elevated levels of kidney injury (measured as elevated protein in the urine, or proteinuria) were identified in the dasatinib patients, with 10% of participants exhibiting severe levels that were later seen to decrease upon cessation of dasatanib use. No participant in the non-dasatinib cohort showed severe proteinuria. Widespread kidney damage in dasatinib patients was also confirmed by kidney biopsy.
With the help of Dr. Adegbite and researchers at Memorial Sloan Kettering Cancer Center, the lead investigator, Dr. Evren U. Azeloglu, Ph.D., Associate Professor of Medicine (Nephrology), Icahn School of Medicine at Mount Sinai, led the multi-center clinical study to determine the incidence of kidney injury in patients taking dasatinib.
The investigators examined glomerular injury through urine albumin-creatinine ratio (UACR) in the 82 patients with chronic myelogenous leukemia who had been continuously taking a tyrosine-kinase inhibitor for at least 90 days. They compared mean differences in UACR, in addition to studying proteinuria. In addition to other blood tests, they also described a case study of a patient who experienced nephrotic-range proteinuria while on dasatinib.
Based on these findings, oncologists prescribing dasatinib may be advised to monitor patients for kidney function or proteinuria and engage a nephrologist to assist in their care. It is also likely that the presence of proteinuria in this setting may result in changes in therapy, especially if proteinuria is severe. Furthermore, the researchers say that these findings suggest that current clinical care, screening guidelines, and Food and Drug Administration guidance on adverse events may need to be updated.
“Ten percent of patients in our study exhibited severe levels of kidney injury, which is striking. Earlier clinical trials reported proteinuria, or high levels of protein in the urine, in less than one percent of patients only, which may be due to their shorter follow-up. Larger prospective clinical studies can help us identify which patients are susceptible to this type of injury and design alternative treatment strategies,” says Dr. Azeloglu.
“We think our study will impact clinical practice significantly, changing standard of care and possibly introducing new black box warnings for the drug that is being studied.”
More information:
Benjamin O. Adegbite et al, Patient-Specific Pharmacokinetics and Dasatinib Nephrotoxicity, Clinical Journal of the American Society of Nephrology (2023). DOI: 10.2215/CJN.0000000000000219
Journal information:
Clinical Journal of the American Society of Nephrology
Source: Read Full Article