Eli Lilly CEO: ‘We got a promising growth picture for the future’
Eli Lilly CEO David Ricks argues his company has ‘one of the freshest portfolios of products’ in the pharmaceutical industry amid the acquisition of Prevail Therapeutics.
Eli Lilly CEO David Ricks told “Mornings with Maria” on Tuesday that the pharmaceutical company’s coronavirus antibody treatment is “now available coast to coast” and it “could help offload the stress on our hospital system right now.”
Continue Reading Below
Ricks added that Eli Lilly’s monoclonal antibody therapy bamlanivimab, which was granted an emergency use authorization by the Food and Drug Administration last month, is a “key to fighting COVID until we have universal vaccinations.”
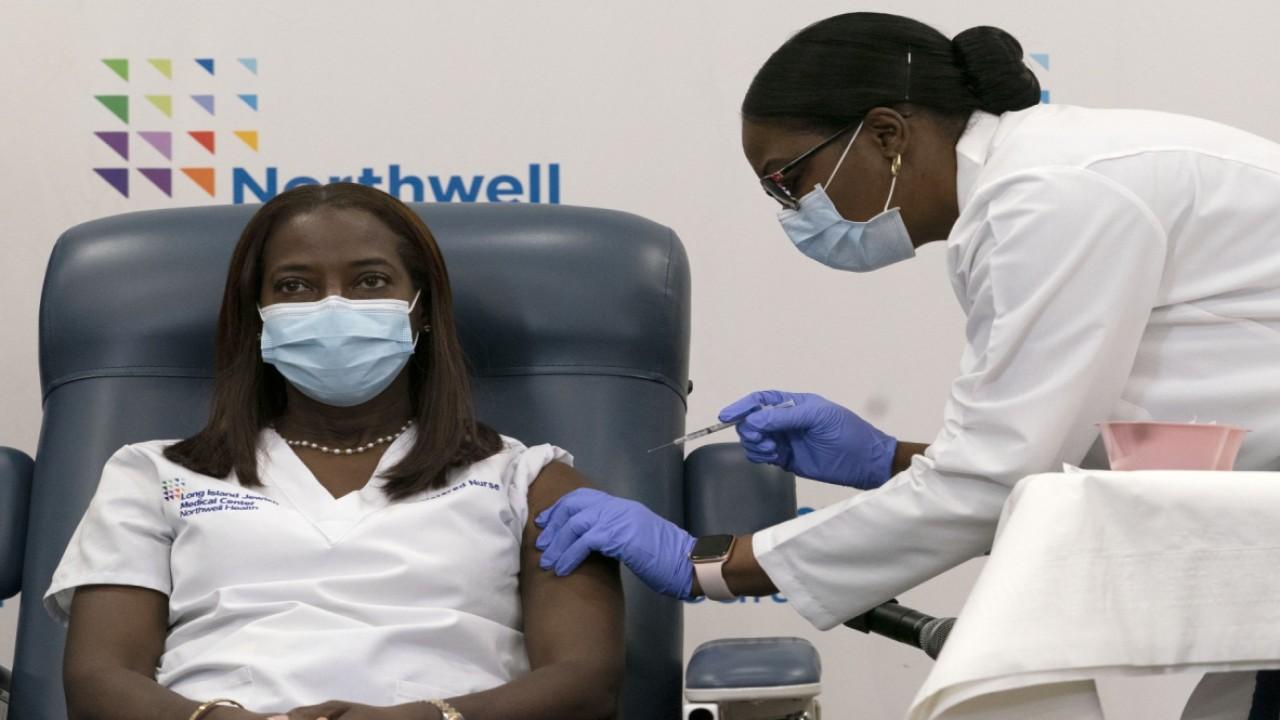
Ricks made the comments as the country’s first COVID-19 vaccine started to arrive in states after the U.S. government gave the final go-ahead to the shots needed to end the outbreak that has killed more than 300,000 Americans.
The U.S. has been reporting new record highs in coronavirus-related hospitalizations recently, according to data provided by The COVID Tracking Project, and hospital systems across the country warned about nearing or reaching full capacity.
“Our scientists went to work in the early days of the pandemic and at Lilly, we repurposed a medicine called olumiant, which is now being used successfully in the hospital on top of remdesivir to save lives,” Ricks explained.
HOW DO THE MODERNA AND PFIZER CORONAVIRUS VACCINES COMPARE?
He added that Eli Lilly also created the “brand new medicine” bamlanivimab, which he said “is an antibody treatment you get right as you get diagnosed” with COVID-19.
The treatment will be used for mild-to-moderate COVID-19 cases in adults who are high-risk, including those 65 years of age or older, or who have certain chronic medical conditions, and children who are 12 years or older and weight at least 88 pounds.
While Eli Lilly has been granted an emergency use authorization by the FDA for bamlanivimab, Dr. Patrizia Cavazzoni, acting director of the FDA’s Center for Drug Evaluation and Research, noted that the agency will "continue to evaluate new data on the safety and efficacy of bamlanivimab as they become available.”
While the safety effectiveness of this investigational therapy continues to be evaluated, bamlanivimab was shown in clinical trials to reduce COVID-19-related hospitalization or emergency room visits in patients at high risk for disease progression within 28 days after treatment when compared to a placebo.
The drugmaker's Phase 2 trial involved 452 patients. Of 309 study participants injected with Eli Lilly’s drug, five, or 1.6%, were later hospitalized or visited the emergency room, compared with nine out of 143 people who received a placebo, or 6.3%.
Bamlanivimab should be administered via a single intravenous infusion as soon as possible after a positive COVID-19 test and within 10 days of symptom onset. It is not authorized for patients who are hospitalized due to COVID-19 or require oxygen therapy due to COVID-19.
Following the emergency use authorization, Eli Lilly immediately started shipping bamlanivimab to national distributor AmerisourceBergen, which will distribute it as directed by the U.S. government's allocation program.
“What we’ve done is taken antibodies from a patient and made it into a medicine, modified it, engineered it and it’s now available coast to coast,” Ricks told host Maria Bartiromo on Tuesday.
The U.S. government has purchased 300,000 doses of bamlanivimab for $375 million.
The company anticipates manufacturing up to 1 million, 700-milligram doses of bamlanivimab by the end of the year, with use around the world through early next year after discussions with global regulators. Beginning in the first quarter of 2021, the supply of Lilly's antibody therapy is expected to increase substantially, as additional manufacturing resources come online throughout the year.
On Tuesday, Ricks addressed “some reporting” that bamlanivimab is “only available for the elite.”

“That is not true,” Ricks said. “We’ve distributed hundreds of thousands of doses to hospitals around the country.”
He added that in Eli Lilly’s announcement of 2021 financial guidance and updates to 2020 guidance released on Tuesday, the company revealed further distribution of bamlanivimab.
FDA PANEL REVIEWS 1ST NEW ALZHEIMER’S DRUG IN 2 DECADES
According to Eli Lilly’s 2021 financial guidance released on Tuesday, 2021 revenue is expected to be between $26.5 billion and $28.0 billion, “driven by volume growth from key growth products” as well as an estimated $1 billion to $2 billion of revenue from COVID-19 therapies.
Earnings per share for 2021 are expected to be in the range of $7.25 to $7.90 and $7.75 to $8.40 on a non-GAAP basis, the company announced.
“Next year we’re projecting our core business will be growing double digits,” Ricks told Bartiromo on Tuesday, nothing that that includes a price drag.
He went on to note that “volume growth is in the mid-teens,” which he said is “boosted by the performance really of our core newest products,” including Trulicity, a diabetes medication and Verzenio, for the treatment of breast cancer.
He noted that “those products had positive additional data readouts just in the last few months and are fueling their growth.”
Ricks also noted that there are “new products coming,” including tirzepatide, which is another diabetes product “similar to Trulicity.”
“We got a promising growth picture for the future,” Ricks said, noting that it is “really driven by a new set of products.”
“Lilly has one of the freshest portfolios of products in pharma and it’s an exciting time for science,” he continued. “It’s really a good moment for the company.”
On Monday, the board of directors of Eli Lilly announced a 15 percent increase in its quarterly dividend to 85 cents a share.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| LLY | ELI LILLY & COMPANY | 166.09 | +8.23 | +5.21% |
| PRVL | PREVAIL THERAPEUTICS INC. | 22.81 | +10.31 | +82.48% |
 |
On Thursday, Eli Lilly announced a definitive agreement to acquire gene therapy company Prevail Therapeutics Inc. for $22.50 per share in cash. Shares of Prevail nearly doubled following the announcement.
The company said the acquisition “will establish a gene therapy program at Lilly, anchored by Prevail's portfolio of neuroscience assets, and will broaden Lilly's commitment to use novel modalities to attempt to address otherwise fatal genetic forms of neurodegenerative disease."
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Speaking to Bartiromo on the same day of the announcement Ricks said, “neurodegenerative diseases, like Alzheimer’s and Parkinson's have been a core to our research strategy for some time so it’s a great fit.”
He added that Eli Lilly is “excited to get to work with them” to bring “new therapies to market.”
CLICK HERE TO READ MORE ON FOX BUSINESS
FOX Business’ Lucas Manfredi and the Associated Press contributed to this report.
Source: Read Full Article






