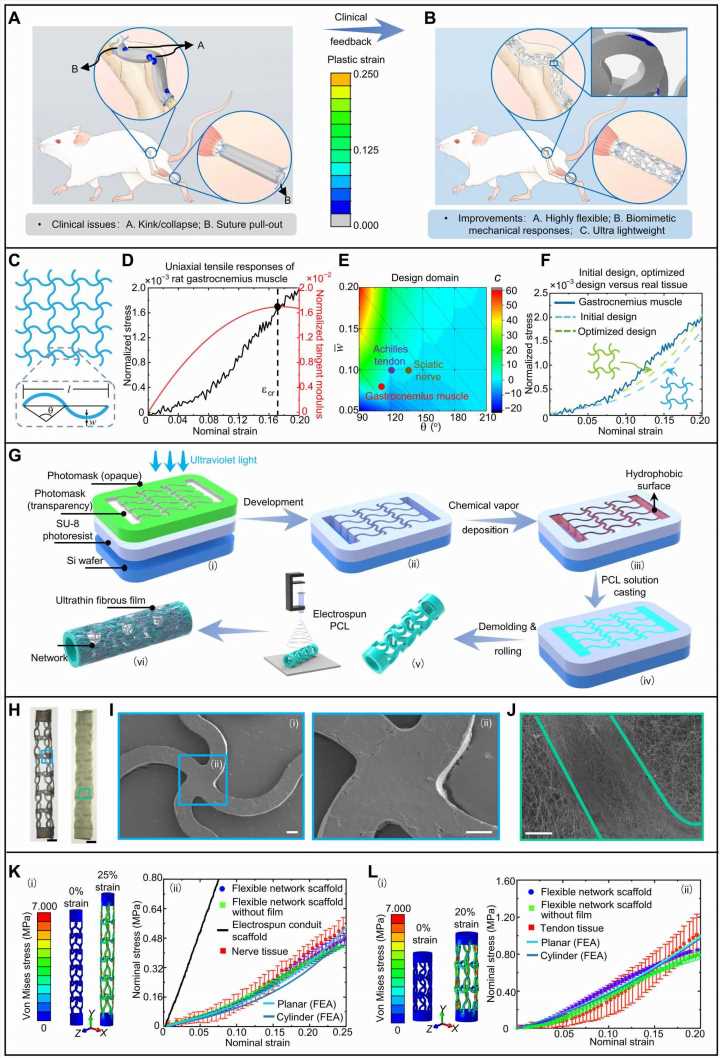
![Inverse design concept and fabrication techniques of flexible network scaffolds for soft tissue regeneration. Schematic illustration of electrospun conduit scaffolds (A) and flexible network scaffolds (B) implanted at the defected sciatic nerves and Achilles tendons. (C) A representative configuration of rectangular SNM (3 × 4 unit cells) and the detail geometric a curved beam. (D) Representative normalized stress-strain curve of rat gastrocnemius muscle and the corresponding tangent modulus versus strain. (E) Contour plot of the phenomenological parameter c with respect to key geometric parameters of curved microstructure. Geometric parameters used in the flexible network scaffolds for sciatic nerve, Achilles tendon and gastrocnemius muscle are marked by circles in the plot. (F) Comparisons of normalized stress-strain curves among target biological tissue, FEA of initial network design, and the optimized one. (G) Fabrication process for flexible network scaffold, (i) exposure; (ii) development; (iii) chemical vapor deposition; (iv) PCL solution casting; (v) demolding and curling; and (vi) electrospinning. (H) Optical images of flexible network scaffold (for sciatic nerves) before and after electrospinning of PCL nanofibrous ultrathin film. (I) SEM image for a central connection in a flexible network scaffold without PCL film (i) [blue-box region in (H)] and magnified views of the connection (ii). (J) SEM image for a segment of curved microstructure with PCL film [cyan-box region in (H)]. (K) Initial and deformed configurations of tubular network scaffold for sciatic nerve obtained from FEA (i). The right presents stress-strain curves of planar and tubular networks obtained from FEA, fabricated planar network structure with and without ultrathin PCL film, electrospun conduit scaffold, and real sciatic nerve (ii). (L) Similar results for the design of network scaffold to reproduce stress-strain curves of Achilles tendon. Scale bars, 800 μm (H) and 100 μm (I and J). Photo credit: S.C., Tsinghua University. Credit: Science Advances, doi: 10.1126/sciadv.adi8606 Bioinspired flexible network scaffolds for soft tissue regeneration](https://scx1.b-cdn.net/csz/news/800a/2023/bioinspired-flexible-n.jpg)
During synthetic scaffold implantation in a clinical setting, graft-host mechanical mismatch is a long-standing issue for soft tissue regeneration. While bioengineers have denoted numerous efforts to resolve this challenge, the regenerative performance of the synthetic scaffolds can be limited by slow tissue growth conditions when compared to autografts, alongside mechanical defects.
In a new report in Science Advances, Shunze Cao and a team of scientists in Engineering, Mechanics, and Orthopedics surgery at the Tsinghua University in China, developed a class of flexible network scaffolds to precisely engineer non-linear mechanical responses. The resulting tissue scaffolds increased tissue regeneration through reduced graft-host mechanical mismatch.
They included a tubular network frame with a flexible network scaffold, with curved microstructures to build the desired mechanical properties and offer a suitable microenvironment for cell growth. The scientists used rat models with sciatic nerve defects or Achilles tendon injuries to show the superior regenerative performance that exceeded the clinically approved electrospun conduit scaffolds.
Bioengineering tissue scaffolds suited for pre-clinical translation
Soft tissue injuries of nerve and blood vessels, tendons, ligaments, and cartilage represent a significant health issue worldwide. Clinically, autografts and allografts are commonly-adopted clinical surgical plans that are in clinical use to treat peripheral nerve defects while facing notable limitations such as limited sources and ethical issues.
Artificial scaffolds for soft tissue regeneration can readily bypass these limits to obtain promising outcomes of regeneration. However, the translational potential of biocompatible scaffolds remain challenging for tissue regeneration due to the mechanical mismatch between the high stiffness of artificial scaffolds and the recipient tissues.
Biologist Lecuit stated that “where shape is at play, forces will function in every single instance.” In this work, Cao and colleagues introduced a rational design method for a class of biomimetic, and flexible network scaffolds to replicate nonlinear mechanical responses of tissues, and guide tissue regeneration through greater biocompatibility of the graft and host.
The researchers developed a flexible network and implanted the scaffold in animal experiments with rat sciatic nerve and Achilles tendon defect models to obtain promising results for soft tissue repair in the clinic.
Rational engineering designs to create flexible network scaffolds
The flexible network scaffold contained a tubular soft network structure and an ultrathin nanofibrous film developed at the outer surface of the tubular network. They introduced an inverse-engineering design developed on a model of soft network materials with curvy microstructures.
They then engineered the flexible network scaffolds by combining lithography, chemical vapor deposition and electrospinning to complete the development of a bilayer network scaffold. Using the constructs, they studied the development of curved microstructures for soft tissue regeneration. Then by using finite elemental analysis and in-lab experiments the team tested the mechanical responses of the flexible network scaffolds.
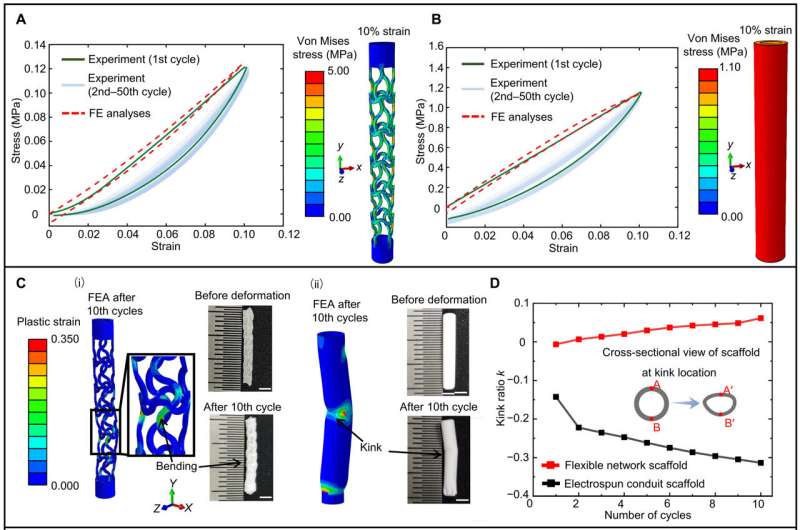
Mechanical behavior of the flexible network scaffolds
The mechanical mismatch between recipient tissue and electrospun conduit scaffolds can result in ineffective regeneration, due to tensile stress on the suture. To assess this effect, they conducted uniaxial tensile responses of flexible network scaffolds in comparison to electrospun conduit scaffolds.
The flexible network scaffold showed much lower risk of suture pull-out than the electrospun conduit scaffold due to much smaller tensile stress. The network scaffolds showed excellent flexibility and a capacity to retain the patency of the luminal conduit, without mechanical failure during the regeneration experiments.
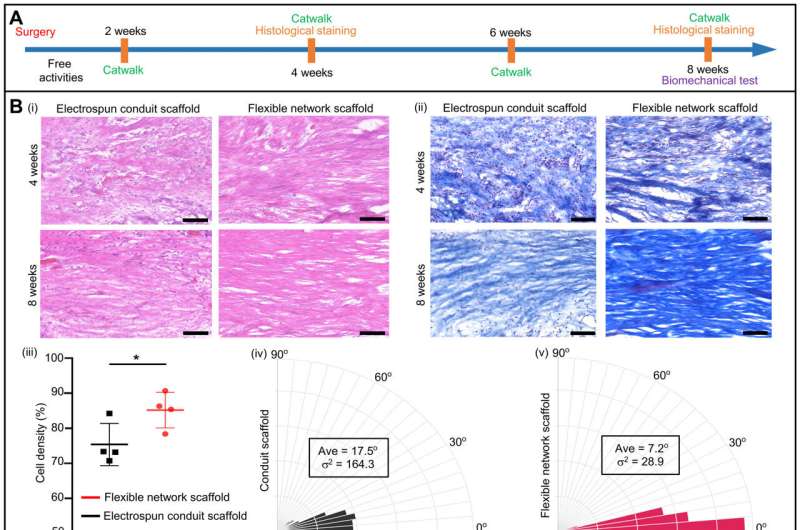
Validating the regenerative performance of the tissue scaffolds
The study team validated the regenerative performance of the flexible scaffold by conducting experiments using rat models with long defects in sciatic nerves.
Aside from the flexible network scaffolds, the team tested electrospun conduit scaffolds and lattice scaffolds with straight microstructures and autologous nerve grafts. The experiments included histological, biomechanical, or functional analyses. Nerve regeneration also accompanied blood vessel regeneration for enhanced regenerative performance of the flexible network scaffolds compared to the electrospun conduit scaffolds.
The flexible network scaffolds showed extensive applications during soft tissue regeneration in a rat Achilles tendon defect model with flexible network and electrospun conduit scaffolds. While the regenerated fibers underwent a remodeling process with the flexible network scaffold, most of the collagen converted from types III to type I. The scientists studied the function of regenerated tendons by conducting quantitative analysis with CatWalk to represent walking videos of rats with Achilles tendon defects repaired post-operatively by two-weeks.
Outlook
In this way, Shunze Cao and colleagues implemented rational reverse-engineering design and biomimetic flexible network scaffolds to enhance the efficiency of soft tissue regeneration. The lightweight, highly flexible, and mechanically biomimetic features of the network scaffolding are appealing for clinical treatment of soft tissue injuries.
The design platform provided a fundamental tool to study and understand the relationship between mechanical microenvironments and biological responses, alongside biological responses of cells and tissues. The team was able to combine experimental investigations with bioinformatics analyses to reveal molecular mechanisms underlying the pro-regeneration effects of biomimetic flexible network scaffolds.
More information:
Shunze Cao et al, Inversely engineered biomimetic flexible network scaffolds for soft tissue regeneration, Science Advances (2023). DOI: 10.1126/sciadv.adi8606
Sean V. Murphy et al, Opportunities and challenges of translational 3D bioprinting, Nature Biomedical Engineering (2019). DOI: 10.1038/s41551-019-0471-7
Journal information:
Nature Biomedical Engineering
,
Science Advances
Source: Read Full Article