A recent study posted to the bioRxiv* preprint server illustrated that specific anti-chemokine antibodies aid in recognizing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent individuals with a desirable disease course.
 Study: Anti-chemokine antibodies after SARS-CoV-2 infection correlate with favorable disease course. Image Credit: Corona Borealis Studio / Shutterstock
Study: Anti-chemokine antibodies after SARS-CoV-2 infection correlate with favorable disease course. Image Credit: Corona Borealis Studio / Shutterstock
Background
Gender, obesity, ethnicity, autoantibodies against interferon, genetic predisposition, and comorbidities such as diabetes and coronary heart disease are some variables that predispose individuals to SARS-CoV-2-related hospitalization and mortality. Coronavirus disease 2019 (COVID-19)-recovered individuals frequently experience months-long symptoms, termed post-acute sequelae of COVID (PASC) or long COVID.
Although anti-SARS-CoV-2 antibodies are protective, those that target interferons and similar immune components are linked to COVID-19 complications. According to a recent yeast display-based high-throughput secretome analysis, COVID-19 results in autoantibodies against many immune components, including chemokines. However, the presence of anti-chemokine antibodies was reported uncommonly by this approach. Moreover, there was no link with long COVID or disease severity, nor was any information on the durability of such autoantibodies with time.
About the study
In the current study, the researchers developed a peptide-based approach to identify and quantify antibodies that attach to a functional area in the 43 human chemokines in a heterogeneous cohort infected with SARS-CoV-2 in 2020.
They examined cells harboring the CC chemokine receptor 2 (CCR2), a critical facilitator of monocyte trafficking into the lung in inflammation and infection, to determine if COVID-19 triggers antibodies that block immune cell migration. Three CCR2 agonists (CC motif chemokine ligand 8 (CCL8), CCL7, and CCL2) were tested in vitro in the vicinity of immunoglobulin Gs (IgGs) isolated from the plasma of 24 COVID-19 recovered individuals or eight virus-free controls.
Further, the researchers reasoned that bioactive antibodies would most likely target chemokines' N-terminal loop (N-loop). They employed enzyme-linked immunosorbent assays (ELISA) to assess plasma samples taken on average six months (t=6m) following illness start from a varied SARS-CoV-2 infection cohort to explore this hypothesis.
Besides, the team created peptides that corresponded to the N-loop of all human chemokines to investigate thoroughly anti-chemokine antibodies in SARS-CoV-2 infection. Antibody concentrations in serial plasma dilutions were evaluated using ELISA, and the signal was shown as a heatmap. In addition, the investigators gathered data from the group at t=12m to see if a specific trend of anti-chemokine antibodies at t=6m was indicative of the duration of symptoms.
Finally, they assessed the significance of anti-chemokine antibodies in conditions other than COVID-19. The scientists analyzed the anti-chemokine antibodies' presence in plasma from 24 patients having chronic human immunodeficiency virus 1 (HIV-1) infection and 13 ankylosing spondylitis (AS), 13 rheumatoid arthritis (RA), and 13 Sjögren syndrome (SjS) affected individuals.
Results
The scientists observed that following COVID-19, autoantibodies targeting chemokines were ubiquitous and that greater levels of particular anti-chemokine antibodies were linked to better illness outcomes. Therefore, the present findings differ from prior studies that correlated autoantibodies to severe SARS-CoV-2 infection.
Autoantibodies to the chemokine C-X-C motif ligand 8 (CXCL8), CXCL5, and CCL25 were higher in SARS-CoV-2 patients with milder illness versus those who needed hospitalization. As these chemokines draw neutrophils and other cells that stimulate tissue remodeling and inflammation, the existence of matching autoantibodies implies that they might play a protective function by decreasing the detrimental inflammatory response correlated with severe COVID-19.
Autoantibodies to three additional chemokines (CCL13, CXCL16, and CXCL21) were enhanced in individuals without PASC one year following infection, similar to those linked with milder illness. These chemokines were crucial for tissue trafficking and B and T lymphocyte activation. As a result, the matching autoantibodies may influence COVID-19's long-term outcome by blocking or altering these cells' recruitment, retention, and activation throughout the immune response.
The anti-CCL22, CCL17, and CXCL19 antibodies presence typically associated with SARS-CoV-2 infection, irrespective of illness trajectory. CCL19 IgG autoantibodies were found early in the acute period but not the CCL17 or CXCL22, indicating that they were probably pre-existing or rapidly formed upon infection. Furthermore, between six and 12 months after the commencement of the illness, antibody titers to CCL19 and various chemokines kept rising.
HIV-1 patients and individuals with the assessed autoimmune diseases had anti-chemokine antibodies, but their trends differed from one another and COVID-19 patients. Antibodies to either CCL17 or CXCL19, a distinctive feature of COVID-19, were not induced by HIV-1 infection.
Additionally, the scientists discovered and described the first human-stemmed monoclonal antibodies to four chemokines, including anti-CXCL16 and anti-CXCL13 antibodies, which were significant for long COVID. All N-loop antibodies tested successfully inhibited chemotaxis, confirming the two-step paradigm of chemokine receptor activation.
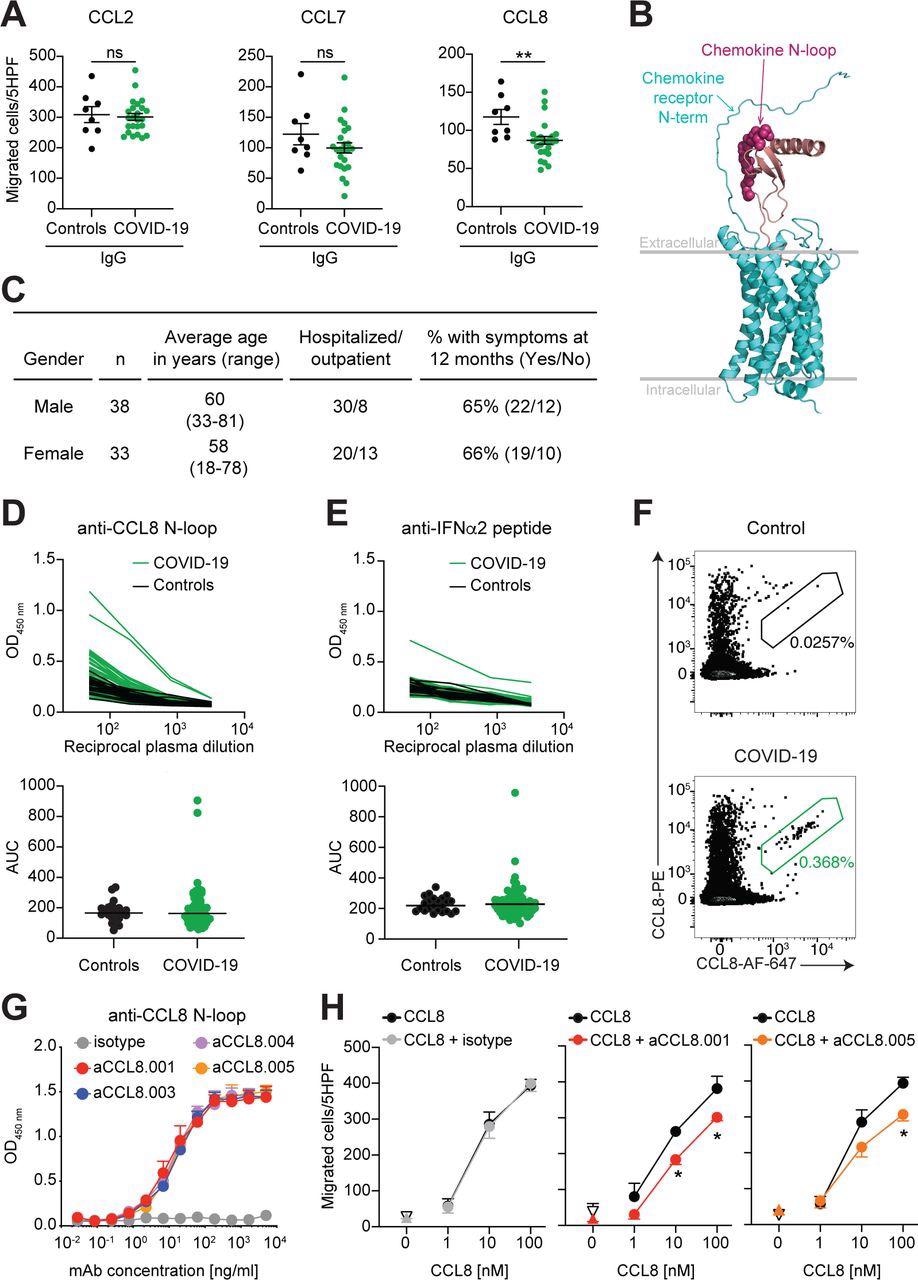
Human monoclonal antibodies that impede CCL8 chemotaxis. (A) IgGs from COVID-19 convalescents inhibit CCL8 chemotaxis. Chemotaxis of CCR2 expressing preB 300.19 cells towards the indicated chemokines was measured in the presence of plasma IgGs from COVID-19 convalescents (n=24) or controls (n=8). Technical triplicates (Mean±SEM) of migrated cells in 5 high-power fields (HPF). Two-tailed Mann–Whitney U- tests. (B) Model of the interaction between a chemokine and its receptor. Arrows point to the area of putative interaction between the N-terminus of the receptor and the chemokine N-loop (shown by spheres). Chemokine is magenta and the chemokine receptor is cyan. (C) Characteristics of the COVID-19 cohort. (D) Identification of individuals with high anti-CCL8 antibodies. Top, optical density (OD450) shows plasma IgG reactivity to the CCL8 N-loop peptide, as determined by ELISA. Bottom, the area under the curve (AUC) of the data in the top panel. Average of two independent experiments. COVID-19 convalescents (n=71); controls (n=23). Horizontal bars indicate median values. (E) Detection of anti-IFNα2 IgGs. Data are shown as in (D). Average AUC from two independent experiments. (F) CCL8 binding human B cells. Flow cytometry plots identify human B cells binding to the CCL8 N-loop peptide (gate). The frequency of antigen-specific B cells is shown. (G) Monoclonal antibodies to the CCL8 N- loop. ELISA binding curves of representative antibodies. Average of two independent experiments (Mean+SEM). (H) Chemotaxis of human monocytes towards CCL8 is inhibited by monoclonal antibodies. Mean±SEM of migrated cells in 5 high-power fields (HPF). At least 3 independent experiments with cells from different donors. Up-pointing triangle is antibody alone, and the down-pointing triangle is buffer control. Two-way RM ANOVA followed by Šídák’s multiple comparisons test.
Conclusions
Overall, the study data revealed antibodies targeting particular chemokines were widespread following SARS-CoV-2 infection, linked to a favorable prognosis, and were indicative of a lack of PASC symptoms one year after infection. Anti-chemokine antibodies were also seen in autoimmune conditions and HIV-1 infection, albeit they address other chemokines than those in SARS-CoV-2 infection.
Finally, monoclonal antibodies obtained from COVID-19-recovered subjects attaching to the N-loop of chemokine hindered leukocyte migration. Considering the importance of chemokines in immune cell trafficking, naturally occurring anti-chemokine antibodies linked with better COVID-19 might be advantageous in moderating the inflammatory response and lowering the development of PASC and hence carry therapeutic potential.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Anti-chemokine antibodies after SARS-CoV-2 infection correlate with favorable disease course; Jonathan Muri, Valentina Cecchinato, Andrea Cavalli, Akanksha A. Shanbhag, Milos Matkovic, Maira Biggiogero, Pier Andrea Maida, Chiara Toscano, Elaheh Ghovehoud, Gabriela Danelon-Sargenti, Tao Gong, Pietro Piffaretti, Filippo Bianchini, Virginia Crivelli, Lucie Podesvova, Mattia Pedotti, David Jarrossay, Jacopo Sgrignani, Sylvia Thelen, Mario Uhr, Enos Bernasconi, Andri Rauch, Antonio Manzo, Adrian Ciurea, Marco Bruno Luigi Rocchi, Luca Varani, Bernhard Moser, Marcus Thelen, Christian Garzoni, Alessandra Franzetti-Pellanda, Mariagrazia Uguccioni, Davide F. Robbiani. bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.23.493121, https://www.biorxiv.org/content/10.1101/2022.05.23.493121v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Ankylosing Spondylitis, Antibodies, Antibody, Antigen, Arthritis, Autoantibodies, CCL19, CCL2, CCL25, Cell, Cell Migration, Chemokine, Chemokines, Chronic, Coronary Heart Disease, Coronavirus, Coronavirus Disease COVID-19, covid-19, CXCL13, Cytometry, Diabetes, Enzyme, Flow Cytometry, Frequency, Genetic, Heart, Heart Disease, HIV, HIV-1, Immune Response, Immunodeficiency, Immunoglobulin, in vitro, Inflammation, Interferon, Interferons, Leukocyte, Ligand, Lymphocyte, Monocyte, Mortality, Neutrophils, Obesity, Peptides, Receptor, Respiratory, Rheumatoid Arthritis, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spondylitis, Syndrome, T Lymphocyte, Virus, Yeast

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article