A multidisciplinary team led by researchers from the Spanish Research Council (CSIC) identifies the genomic cellular map associated with hippocampal sclerosis, a major histopathological condition of temporal lobe epilepsy. The study, published in Cell Reports, identifies cell-type specific transcriptional signatures of hyper-excitability and neurodegeneration, providing grounds for improved diagnosis. While the presence of sclerosis is essential for identifying temporal lobe epilepsy (the most common form of drug-resistant epilepsy), it is also detected in some cases of dementia associated with Alzheimer’s disease.
Hippocampal sclerosis is characterized by the loss of specific neuronal populations and exacerbated activation of other cell types resident in the brain, such as microglia and astrocytes. However, understanding why some neuronal types are more vulnerable than others has remained largely unclear.
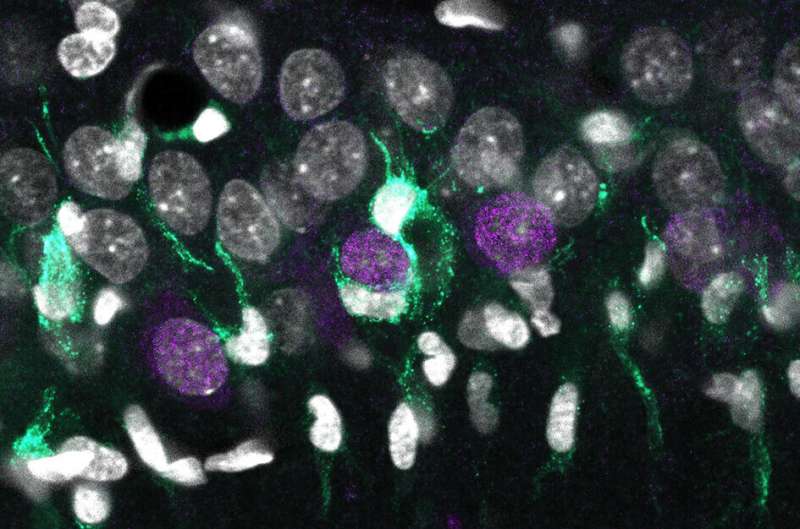
In this work, the researchers have developed a transcriptional map that identifies the precise genetic code of all cell types specifically affected in the disease. “With these maps we seek to match different genes with specific biological elements. In the case of the brain, we aim linking expression of some specific genes with different cell types, such as neurons, astrocytes and microglia,” explains Liset Menéndez de la Prida, a scientist at the Cajal Institute of the CSIC, who lead the study together with José López-Atalaya, from the Institute of Neurosciences (CSIC-UMH) in Alicante.
To do this, researchers applied two advanced procedures for biological sampling: Laser micro-dissection of localized brain tissue and sorting of individual cell nuclei extracted from rats and mice. Using cutting-edge genomic and bioinformatic approaches allowed them to identify specific genetic signatures of degenerative processes.
“When neurons begin to degenerate, they release some signals that can be detected by microglia, which are mediating the inflammatory and neurotoxic response. Each of these processes involve the activation or inactivation of some genetic programs defining a specific genetic signature. We identify some of these genetic signatures associated to neuronal types,” explains López-Atalaya.
Researchers also recorded the electrical activity of individual neurons in vivo and found signs of epileptiform activities in some of them. These more hyperexcitable neurons tended to be located in a specific sublayer within the hippocampal region (the superficial sublayer) and expressed some characteristic genes. “Very strikingly, we found that the hyperexcitability map perfectly matched neurodegeneration signatures,” adds de la Prida.
To ease dissemination amongst the neuroscience and clinical communities, researchers have made the data associated with this study accessible as a public resource though two interactive websites.
Temporal lobe epilepsy and Alzheimer’s
Temporal lobe epilepsy is associated with many causes, such as brain infection, injuries caused by trauma, tumors or various genetic factors associated with anatomical changes of the laminar organization of the cerebral cortex. Something common to this heterogeneous group of disorders is the origin of seizures in the temporal lobe, associated with hippocampal atrophy and sclerosis.
In Alzheimer’s disease, which affects similar structures of the temporal lobe, electrographic seizures comparable to those of temporal lobe epilepsy have been described. Both diseases are associated with episodic memory deficits, which involve the hippocampus. In some cases of Alzheimer’s, atrophy of the hippocampus is also observed, so the results of this study could be of relevance to identify their underlying commonalities.
Source: Read Full Article